Irbesartan
Inquiry
| Post Date: | Apr 05,2025 |
| Expiry Date: | Oct 02,2025 |
| Detailed Description: |
Cas No. :138402-11-6
Specs:USP/EP Product IRBESARTAN CAS NO;138402-11-6 Standard;USP MF;C25H28N6O MW;428.53 Specification: Test Items Standard Result Description White crystalline powder Complies Solubility Slightly soluble in alcohol and in methylene chloride, practically insoluble in water Complies Identification:IR/HPLC Similar to standard Complies Water ≤0.5% 0.29% Residue on ignition ≤0.1% 0.04% Azide ≤0.001% Complies Heavy metal ≤0.002% Complies Related compounds Total compounds ≤0.5% 0.31% Releated compounds A ≤0.2% 0.05% Any other compound ≤0.1% 0.04% Assay 98.0%-102.0% (on anhydrous basis) 99.5% Residual solvents Ethanol≤5000ppm Not detected Toluene≤890ppm 75ppm Dichloromethane≤600ppm Not detected N,N-Dimethylformamide≤880ppm Not detected t-Butyl methyl ether≤5000ppm Not detected Conclusion It complies with all the requirements of USP35 Patent Disclaimer: 1. Products protected by valid patents are not offered for sale in jurisdictions where the sale of such products constitutes a patent infringement. The current list only reflects the products and technologies that are available, under development: note that some products may be developed or produced for internal and experimental uses with no commercal aim. 2. Sales of products are limited to those allowed by Chapter VII PLPRC 63, the above includes Research and development quantities. 3. R&D use in accordance with (1) 35 USC 271(e)+A13(1) in the U.S.; (2) Section 69.1 of Japanese Patent Law in Japan; (3) Section 11, No. 2 of the German Patent Act of 1981 in Germany; (iv) Section 60, Paragraph 5b of the U.K. Patents Act of 1977 in the U.K.; (4) Sections 55.2(1) and 55.2(6) and other common law exemptions of Canadian patent law; (5) Section 68B of the Patents Act of 1953 in New Zealand together with the amendment of same by the Statutes Amendment Bill of 2002; (6) such related legislation and/or case law as may be or become applicable in the aforementioned countries; and (7) such similar laws and rules as may apply in various other countries. |
| CAS Registry Number: | 138402-11-6 |
| Synonyms: | ;Irbesartan (GMP);3-butyl-2-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]-2,4-diazaspiro[4.4]non-3-en-1-one;2-BUTYL-3-[[2'-(1H-TETRAZOL-5-YL)[1,1'-BIPHENYL]-4-YL]METHYL]-1,3-DIAZASPIRO[4.4]NON-1-EN-4-ONE;BMS-186295;AVAPRO;APROVEL;SR-47436;2-butyl-3-{[2'-(2H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one; |
| Molecular Formula: | C25H28N6O |
| Molecular Weight: | 428.5294 |
| Molecular Structure: | 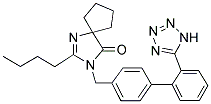
|
| Company: | Taizhou Tianrui Chempharm Co., Ltd. [ China ] |
| Contact: | |
| Tel: | +86-576-88603301 |
| Fax: | +86-576-88603302 |
| Email: | sales@tianruipharm.com |
-
Disclaimer statement:The information and data included above have been realized by the enterprises and compiled by the staff, and are subject to change without notice to you. The Chemnet makes no warranties or representations whatsoever regarding the facticity, accuracy and validity of such information and data. In order to ensure your interest, we suggest you chose the products posted by our gold suppliers or VIP members.

-
- Rifamycin S Sodium
- 3 Formyl Rifamycin SV
- Temozolomide
- Lovastatin
- Trans-4-methyl cyclohexyl ...
- Trans-4-ethylcyclohexylami...
- Trans-4-ethylcyclohexylami...
- 2-phenethyl isocyanate
- N-[(2'-cyano(1.1'-biphenyl...
- Ethyl-2-[[(2'-cyanobipheny...
- Ethyl-2-ethoxy-1-[(2'-(1Ht...
- 2-ethoxy-1-[[(2'-(1-triphe...
- 1-(cyclohexyloxycarbonylox...
- 2-chloroethyl isocyanate
- 2-chloroethyl isocyanate
- GAMMA PICOLINE
- 4-cyano pyridine
- 3-cyano pyridine
- GAMMA PICOLINE
- Isoniazid
- Rifapentine
- Ethambutol HCL
- Isonicotinic acid
- Rifamycin S Sodium
- Rifamycin-S Acid